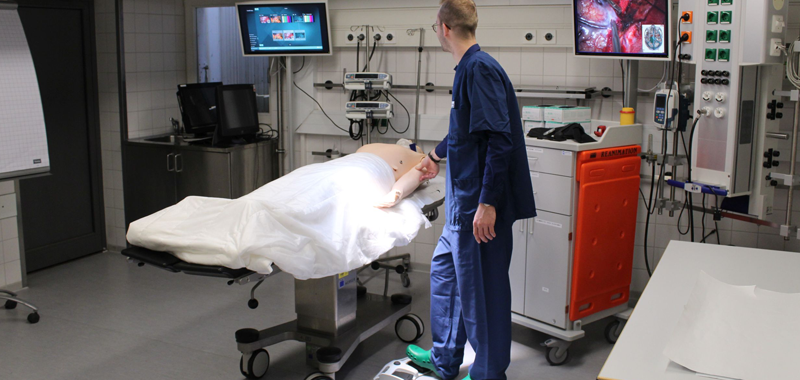
The importance of usability in medical technology has greatly increased due to the new Medical Device Regulation (MDR). A user-oriented product development is a core factor for successful medical products in increasingly complex clinical processes and workflows. That is why the regulations should be seen as an opportunity to shape innovation processes in line with the market through the structured involvement of users.
In this article you will learn briefly:
- what usability is and why it is so important, especially in medical technology
- what is the subject of usability according to the regulations
- why usability is the key to a successful medical device
What is usability?
Usability engineering started in the aerospace industry. Since then, this principle has been implemented in many other areas, including medical technology. Usability describes the quality of use that a user experiences when interacting with technical systems and devices or products.
“… the property of the user-product interface that includes the effectiveness, efficiency, ease of learning and satisfaction of the user. (IEC 62366-1)”
Usability represents an important concept for effectiveness and safety of products in the treatment of patients. The easier it is for the users of a product to realise their goals, the higher the usability of the product and thus the higher the customer satisfaction.
Why usability is so important
At the latest since the announcement of the new MDR, proof of usability has become the focus of attention for manufacturers of medical technology. Whereas at the time of the MDD, studies could be avoided in some cases or carried out on a smaller scale, the MDR increasingly calls for extensive studies to prove usability. In short: without good and measurable usability, hardly any product will be able to enter the market.
What is the subject of usability according to the regulations
In the course of the development of a medical device, the following two essential steps are mandatory components for approval:
Formative Evaluation: A Formative Evaluation already provides possible improvement potentials of the product during product development, which can still be implemented during the development process. In contrast to the Summative Evaluation, the regulatory requirements are much lower here, which unfortunately also leads to the fact that often only internal company meetings or similar are recorded as Formative Evaluations even though it actually has the most potential to have a significant influence on the outcome of the development. An iterative process through expert interviews, workshops and user tests ensures the continuous involvement of the end users and a constant improvement of the product.
Summative Evaluation: Following the completion of the final prototype, a Summative Evaluation is carried out. Here, all (or all security-related) usage scenarios are tested by involving potential future users and a final evaluation is carried out. Summative evaluation is linked to high regulatory requirements, which makes it both time-consuming and expensive. Ideally, good usability should therefore be confirmed and late changes to the product avoided. In addition to the required final report for the usability file, training concepts for end users can also be derived.
Why usability is the key to success
Formative and especially summative usability testing is time-consuming and costly. Involving appropriate clinical end-users (doctors, nurses, cleaning staff, IT administrators, patients, etc.) is a real challenge for medical device manufacturers due to lack of time of clinical staff, compliance issues and finding the right contact person in clinics. At the same time, clinical treatments and interventions, as well as innovations in medical technology, are becoming increasingly complex. Increased involvement of lead users in medical technology innovations is therefore not only a regulatory must. It is often also the key to a user-friendly product that meets practical needs and represents real added value compared to alternative products. Proof of usability therefore forms the basis for an effective, innovative and promising development process. Furthermore, the close cooperation with a large number of clinical experts helps to find early adopters and to convince important KOLs of the product by including their opinions and suggestions.
By expanding the usability process with a few more modules, a holistic and effective innovation process can be established out of regulatory necessity that not only guarantees a successful end product, but also focuses on the needs of many employees involved in the development process and thus increases their effectiveness.
In regards to the SAFETY project usability also plays a key role during simulation for medical practice. Bourges-Waldegg et al. already argued in 2000 that usability issues are of great importance on evaluation programs, as they can have both a positive and a negative impact on teaching-learning processes. The point out that “failure to detect usability problems and to make adequate prescriptions during these programs can consequently have an impact on the development of national curricula, educational goals, contents, policies, etc.”
Summary: Advantages of a good usability process
The increased requirements for usability proof should be seen as an opportunity and benefit from:
- high practical relevance of the innovation by including KOL’s (innovation leadership)
- an efficient innovation process with low error potential
- secure and efficient approval and rapid market entry
- effective and well-founded marketing
- highlighting of advantages in direct comparison with competing products (through so called “benchmarking studies”)
- Bourges-Waldegg, L. Moreno and T. Rojano, “The role of usability on the implementation and evaluation of educational technology,” Proceedings of the 33rd Annual Hawaii International Conference on System Sciences, 2000, pp. 7 pp.-, doi: 10.1109/HICSS.2000.926722.
Written by Robin Strak, LMU München