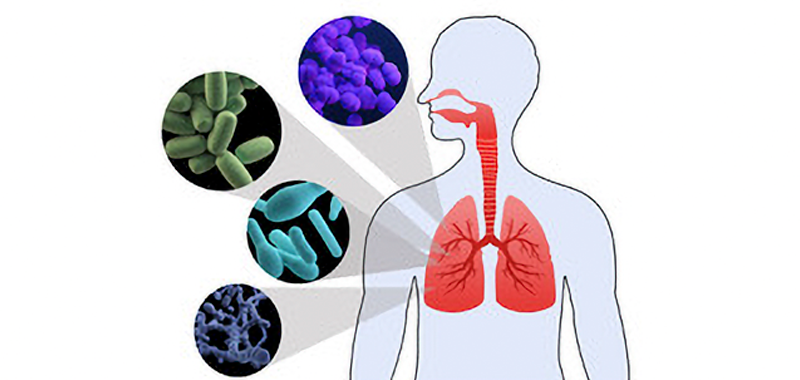
Human microbiota consists mostly of bacteria but also includes viruses, molds, yeasts, and protozoa which colonize skin and mucous membranes of cavities exposed to the exterior, such as the gastrointestinal, respiratory and genitourinary tracts. In these environments, microbiota establishes a symbiotic relationship with the host, providing early modulation of the host’s physiological development, nutrition, immune and pathogen resistance functions in all stages of life. [1,2] Therefore dysbiosis in the microbiota has been associated with diseases as inflammatory bowel disease, multiple sclerosis, diabetes, allergies, asthma, autism, and cancer [3, 4, 5, 6, 7].
Initially lungs and airways were omitted from organs to be studied because there was a common belief that healthy lungs were sterile, but recent data has changed the sterile theory of the lungs: the respiratory tract contains microbial communities from the nasal fossae to the pulmonary alveolus, with the highest concentrations found in the upper airways. [8] [9] This autochthonous microbiota contributes to the defense against colonization and infection by pathogens in the respiratory mucosa and thereby prevents their spread throughout the tract. So, in healthy conditions lungs microbiota consists of a transient community of microorganisms, mainly from the nasopharynx and oropharynx, which settle down in lungs establishing an apparent homeostasis with the host.[10]
It is not clear if microorganisms found in the lungs are unique but most data suggest substantial diversity in healthy individuals. [11, 12, 13, 14]
Recent data has also shown that the presence of specific microbe (e.g. Treponema Whipplei) in the lungs of healthy subjects was not found in upper airway samples and the evidence that colonization of the lower airways with this microbe was enhanced in immunodeficiency state. [15] These findings suggest that unique taxa could find a special niche in the lung environment and exert an immunomodulatory role. [16]
Even if the role of the gut microbiota in shaping the mucosal immune system is clearly understood, it remains unclear whether the observed associations with lung microbiota and immune system are causal or not. [17, 18]
Otherwise, there is a relationship between gut and lung microbiota known as “gut-lung axis” and acting on this connection, pharmacological treatments with probiotics are under study processes to verify if modifiers of gut microbiota can also influence lung microbiota and the pulmonary pathologies, especially in critical hills. [19,20, 21]
On this basis, our research team at the University of Foggia (Italy) aims to study the effects of nutrition treatment in traumatic brain injured patients in the Intensive Care Unit on lung microbiota to seek to better how this new treatment can influence the outcome in these specific patients.
Authors: Antonella Cotoia, Giuseppe Ferrara, Lucia Mirabella, Gilda Cinnella – University of Foggia (Italy)
References:
- Gill, S. R. et al. Metagenomic analysis of the human distal gut microbiome. Science 312, 1355–1359 (2006). DOI: 10.1126/science.1124234
- Dominguez-Bello MG, Godoy-Vitorino F, Knight R, Blaser MJ. Role of the microbiome in human development. Gut. 2019;68:1108—14 https://gut.bmj.com/content/68/6/1108
- Petersen C, Round JL. Defining dysbiosis and its influence on host immunity and disease. Cell Microbiol. 2014;16:1024–33. https://doi.org/10.1111/cmi.12308
- Backhed F, Fraser CM, Ringel Y, Sanders ME, Sartor RB, Sherman PM, et al. Defining a healthy human gut microbiome: current concepts, future directions, and clinical applications. Cell Host Microbe. 2012;12:611–22.
- Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med. 2014;20:159–66. https://doi.org/10.1038/nm.3444
- Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155:1451–63.
- Garrett WS. Cancer and the microbiota. Science. 2015;348:80–6. 1126/science.aaa4972
- Dickson RP, Erb-Downward JR, Huffnagle GB. The role of the bacterial microbiome in lung disease. Expert Rev Respir Med 2013;7:245–57. https://doi.org/10.1586/ers.13.24
- Man WH, de Steenhuijsen Piters WA, Bogaert D. The microbiota of the respiratory tract: gatekeeper to respiratory health. Nat Rev Microbiol. 2017;15:259—70. https://doi.org/10.1038/nrmicro.2017.14
- . Dickson RP, Erb-Downward JR, Freeman CM, McCloskey L, Beck JM, Huffnagle GB, et al. Spatial variation in the healthy human lung microbiome and the adapted island model of lung biogeography. Ann Am Thorac Soc. 2015;12:821—30.
- Morris A, Beck JM, Schloss PD, Campbell TB, Crothers K, Curtis JL, Flores SC, Fontenot AP, Ghedin E, Huang L, et al. Comparison of the respiratory microbiome in healthy non-smokers and smokers. Am J Respir Crit Care Med 2013;187:1067–1075
- Segal LN, Alekseyenko AV, Clemente JC, Kulkarni R, Wu B, Chen H, Berger KI, Goldring RM, Rom WN, Blaser MJ, et al. Enrichment of lung microbiome with supraglottic taxa is associated with increased pulmonary inflammation. Microbiome 2013;1:19.
- Hilty M, Burke C, Pedro H, Cardenas P, Bush A, Bossley C, Davies J, Ervine A, Poulter L, Pachter L, et al. Disordered microbial communities in asthmatic airways. PLoS One 2010;5:e8578.
- ErbDownward JR, Thompson DL, Han MK, Freeman CM, McCloskey L, Schmidt LA, Young VB, Toews GB, Curtis JL, Sundaram B, et al. Analysis of the lung microbiome in the “healthy” smoker and in COPD. PLoS One 2011;6:e16384.
- Lozupone C, CotaGomez A, Palmer BE, Linderman DJ, Charlson ES, Sodergren E, Mitreva M, Abubucker S, Martin J, Yao G, et al. Widespread colonization of the lung by Tropheryma whipplei in HIV infection. Am J Respir Crit Care Med 2013;187:1110–1117.
- Leopoldo N. Segal 1and Martin J. Blaser, A Brave New World: The Lung Microbiota in an Era of Change https://doi.org/10.1513/AnnalsATS.201306-189MG.
- Ivanov II, Frutos Rde L, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, Finlay BB, Littman DR. Specifc microbiota direct the differentiation of IL-17–producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe 2008;4:337–349. https://doi.org/10.1016/j.chom.2008.09.009
- Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 2011;331:337–341. DOI: 10.1126/science.1198469
- Mukherjee S, Hanidziar D. More of the gut in the lung: how two microbiomes meet in ARDS. Yale J Biol Med. 2018;91:143–9 Available from: http://www.ncbi.nlm.nih.gov/pubmed/29955219.
- van Ruissen MCE, Bos LD, Dickson RP, Dondorp AM, Schultsz C, Schultz MJ. Manipulation of the microbiome in critical illness-probiotics as a preventive measure against ventilator-associated pneumonia. Intensive Care Med Exp. 2019;7:37 Available from http://www.ncbi.nlm.nih.gov/pubmed/31346841.
Weng H, Li J-G, Mao Z, Feng Y, Wang C-Y, Ren X-Q, et al. Probiotics for preventing ventilator-associated pneumonia in mechanically ventilated patients: a meta-analysis with trial sequential analysis. Front Pharmacol. 2017;8:717 Available from http://www.ncbi.nlm.nih.gov/pubmed/29062279